Multimedia Gallery
Carbon buckyball's boron cousin -- the 'borospherene'
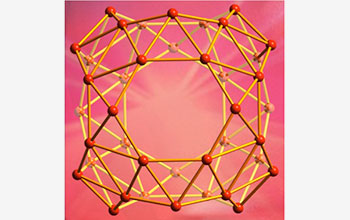
The carbon buckyball's boron cousin the "borospherene," in which a cluster for 40 boron atoms forms a hollow cage-like molecule.
More about this image
Thirty years ago, researchers discovered buckyballs, soccer ball-shaped molecules of carbon, thereby helping to usher in the nanotechnology era. Now, researchers at Brown University and Shanxi and Tsinghua universities in China have shown that a cluster of 40 boron -- carbon’s neighbor on the periodic table -- atoms forms a hollow molecular cage similar to a carbon buckyball. Until now, such a boron structure had only been a theoretical speculation. The researchers call their newfound nanostructure the "borospherene."
"This is the first time that a boron cage has been observed experimentally," said Lai-Sheng Wang, a professor of chemistry at Brown who led the team that made the discovery. "As a chemist, finding new molecules and structures is always exciting. The fact that boron has the capacity to form this kind of structure is very interesting."
The discovery in 1985 of carbon buckyballs -- 60 carbon atoms arranged in pentagons and hexagons to form a sphere that looks similar to a soccer ball -- was soon followed by discoveries of other hollow carbon structures like carbon nanotubes and graphene.
Boron has one less electron than carbon so it can’t form the same 60-atom structure found in the buckyball without collapsing on itself. But Wang and his colleagues showed that clusters of 36 boron atoms form one-atom-thick disks, which might be stitched together to form an analog to graphene, dubbed "borospherene." Wang’s research also suggested that boron clusters with 40 atoms seemed to be abnormally stable compared to other boron clusters. To figure out what a 40-atom cluster would actually look like, Wang and colleagues used a combination of experimental work and modeling using high-powered supercomputers.
Wang’s colleagues used computer simulations to model over 10,000 possible arrangements of 40 boron atoms bonded to each other. The simulations estimated both the shape of the structures and the electron binding energy for each structure, a measure of how tightly a molecule holds its electrons. Next, Wang and colleagues used a technique called photoelectron spectroscopy to test the actual binding energies of boron clusters in the lab to see if they matched any of the theoretical structures generated by the computer. The experiments showed that 40-atom-clusters form two structures with distinct binding spectra, two of which turned out to be dead-on matches with the spectra for two structures generated by the computer models. One was a semi-flat molecule and the other was the buckyball-like spherical cage.
"The experimental sighting of a binding spectrum that matched our models was of paramount importance," Wang said. "The experiment gives us these very specific signatures, and those signatures fit our models."
Borospherene consists of 48 triangles, four seven-sided rings and two six-membered rings. Several atoms stick out a bit from the others, making the surface of borospherene somewhat less smooth than a buckyball.
This research was supported in part by a grant from the National Science Foundation (grant CHE 1263745).
To learn more about this research, see the Brown news story Researchers discover boron 'buckyball'. (Date image taken: 2014; date originally posted to NSF Multimedia Gallery: Aug. 18, 2017)
Credit: Lai-Sheng Wang/Brown University
Images and other media in the National Science Foundation Multimedia Gallery are available for use in print and electronic material by NSF employees, members of the media, university staff, teachers and the general public. All media in the gallery are intended for personal, educational and nonprofit/non-commercial use only.
Images credited to the National Science Foundation, a federal agency, are in the public domain. The images were created by employees of the United States Government as part of their official duties or prepared by contractors as "works for hire" for NSF. You may freely use NSF-credited images and, at your discretion, credit NSF with a "Courtesy: National Science Foundation" notation.
Additional information about general usage can be found in Conditions.
Also Available:
Download the high-resolution JPG version of the image. (178.0 KB)
Use your mouse to right-click (Mac users may need to Ctrl-click) the link above and choose the option that will save the file or target to your computer.