Multimedia Gallery
How Do Carbon Nanotubes Grow? (Image 1)
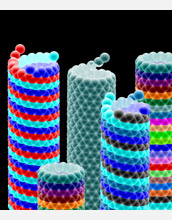
The atomic wall-tapestry of nanotubes depends on their chiral symmetry, or helicity. Not only does this symmetry define all physical properties, but it also controls the rate of self-assembly of carbon atoms, or growth rate. The larger the chiral angle, the more loose thread-ends are available to accept arriving atoms and the faster the tube grows. [See related image Here.] (Date of Image: 2009)
More about this Image
How do carbon nanotubes grow? The intimate mechanisms of carbon nanotube growth have provided scientists and engineers with a compelling puzzle for more than decade. Lack of experiments permitting the direct "viewing" of atomic-scale events--along with seemingly chaotic data from computer simulations, such as molecular dynamics--have added to this complex problem. Professor Boris Yakobson of Rice University and his research team have elucidated novel insight into nanotube growth by using mathematical models.
Nanotubes grow in a twisting pattern as they self-assemble with new atoms attaching to the end as the tube forms. The growth of nanotubes depends on their chiral symmetry, or helicity. One could compare this growth to a roll of gift-wrapping paper that is being rolled up. As the roll of gift-wrapping paper is formed into a tube, excess paper sometimes hangs off the end. As they form, nanotubes appear rolled, or twisted, resulting in an odd angle with the ends "hanging off" where new atoms are attached. This angle is called the nanotube's "chiral" angle. The larger the chiral angle, the more loose-thread ends are available to accept arriving atoms and the faster the tube grows.
The research team's efforts resulted in finding a direct proportional relationship between a nanotube's chiral angle (the amount the nanotube is twisted) and how fast it grows. These results lead to a deeper understanding of growth regularity and to direct quantitative predictions of growth rate, as being proportional to the chiral angle, in comparison to available experimental data.
Credit: M. Bankehsaz and B. I. Yakobson, Rice University; courtesy PNAS
Images and other media in the National Science Foundation Multimedia Gallery are available for use in print and electronic material by NSF employees, members of the media, university staff, teachers and the general public. All media in the gallery are intended for personal, educational and nonprofit/non-commercial use only.
Images credited to the National Science Foundation, a federal agency, are in the public domain. The images were created by employees of the United States Government as part of their official duties or prepared by contractors as "works for hire" for NSF. You may freely use NSF-credited images and, at your discretion, credit NSF with a "Courtesy: National Science Foundation" notation.
Additional information about general usage can be found in Conditions.
Also Available:
Download the high-resolution JPG version of the image. (2.6 MB)
Use your mouse to right-click (Mac users may need to Ctrl-click) the link above and choose the option that will save the file or target to your computer.