News Release 08-117
Radicals Shake Up Molecules in a Tug o' War
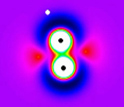
An unexcited deuterium molecule, before being tugged into vibration by a hydrogen atom.
July 3, 2008
View a video representing a collision between a molecule of deuterium and a radical hydrogen atom.
This material is available primarily for archival purposes. Telephone numbers or other contact information may be out of date; please see current contact information at media contacts.
Until now, it was commonly thought that colliding molecules get the shakes as the result of energy transfer solely from the smashing of the molecules, but some new research adds a second means by which colliding molecules become vibrationally excited--it is being called the "Tug o' War Mechanism."
The new experiment, transforming the textbook story, was performed in the lab of Richard Zare, chair of the Department of Chemistry at Stanford University. This work on energy transferring, or inelastic, collisions is featured in the July 3, 2008 issue of the journal Nature.
"How energy transfer occurs in molecular collisions is a topic of deep interest to chemists, for energy transfer is often the precursor to chemical transformations," Zare said. "This is the reason why I regard finding a new mechanism for energizing molecules through collisions to be of such potential importance."
"The work by Zare and his colleagues shows an interesting and unexpected result," shared Charles Pibel, director of the Physical Chemistry Program at the National Science Foundation (NSF). "The conventional wisdom had been that most collisions between molecules excite vibrational motion through a hard impact, like a piano's hammer striking a string."
But instead of molecules impacting and deflecting backwards, the Tug o' War Mechanism stretches the molecule and then releases it, starting the molecule rattling. The effect is "more like a violinist, plucking a string, pizzicato." In the collisions studied by the Zare group, a lone hydrogen atom tugs on one end of a deuterium molecule and lets go, exciting the molecular deuterium into vibration.
More generally, the Tug o' War Mechanism comes into play when a certain type of unstable species, a free radical, looms down a path for collision with another molecule. Because free radicals lack a properly balanced shell of electrons, they are more susceptible to the mechanism than other types of atoms or molecules.
Sometimes a collision between a free radical and another molecule rips apart chemical bonds and atoms are exchanged amongst the colliding partners--that is, a chemical reaction takes place. However, other times when free radicals approach other molecules, this Tug o' War Mechanism stretches the molecule without breaking the bonds.
In this way, free radicals act like renegades attempting to steal atoms from their parent molecule by tugging them away. But if a collision is not at the right angle, the radical might not be able to break the bonds of the molecule. It releases its hold on the nearest atom, or atoms, and then departs. The frustrated, or unsuccessful, chemical reaction nevertheless imparts some energy on the victim of the free radical attack. It is this energy that sets the molecule off dancing with a new vibrational pattern.
The research, funded by NSF, was led by Noah T. Goldberg at Stanford University and Stuart J. Greaves at the University of Bristol, U.K., and included Zare, Jianyang Zhang and Daniel J. Miller, all from Stanford University, and Eckart Wrede from the University of Durham, U.K.
-NSF-
-
View Video
A movie representing a collision between a mildly vibrating deuterium molecule and a hydrogen atom.
Credit and Larger Version
Media Contacts
Brandon Michael Garcia, National Science Foundation, (703) 292-8612, email: bgarcia@nsf.gov
Bobbie Mixon, National Science Foundation, (703) 292-8485, email: bmixon@nsf.gov
Program Contacts
Charles D. Pibel, National Science Foundation, (703) 292-4971, email: cpibel@nsf.gov
Principal Investigators
Richard N. Zare, Stanford University, (650) 723-3062, email: zare@stanford.edu
Related Websites
Zare Lab: http://www.stanford.edu/group/Zarelab/
The U.S. National Science Foundation propels the nation forward by advancing fundamental research in all fields of science and engineering. NSF supports research and people by providing facilities, instruments and funding to support their ingenuity and sustain the U.S. as a global leader in research and innovation. With a fiscal year 2023 budget of $9.5 billion, NSF funds reach all 50 states through grants to nearly 2,000 colleges, universities and institutions. Each year, NSF receives more than 40,000 competitive proposals and makes about 11,000 new awards. Those awards include support for cooperative research with industry, Arctic and Antarctic research and operations, and U.S. participation in international scientific efforts.
Connect with us online
NSF website: nsf.gov
NSF News: nsf.gov/news
For News Media: nsf.gov/news/newsroom
Statistics: nsf.gov/statistics/
Awards database: nsf.gov/awardsearch/
Follow us on social
Twitter: twitter.com/NSF
Facebook: facebook.com/US.NSF
Instagram: instagram.com/nsfgov